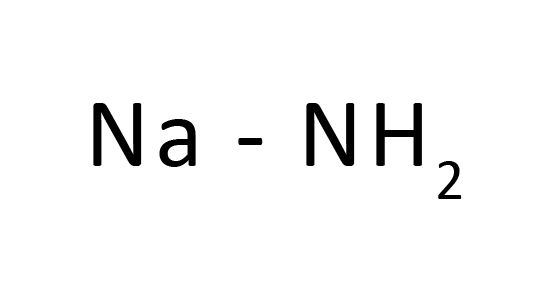Sodium hydride
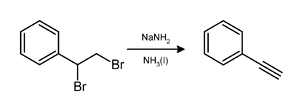
Sodium hydride can be utilized on the other hand as a metallising reagent. The response can be done in THF or benzene, yet at times, improved results are gotten utilizing DMF, which guarantees further developed dissolvability of the intermediates.39 countless subsidiaries have been arranged as of late by utilizing this technique:
Sodium Hydride is by and large quickly accessible in many volumes. High virtue, submicron and nanopowder structures might be thought of. Hydride compounds are utilized frequently utilized as versatile wellsprings of hydrogen gas. American Components produces numerous standard grades while material, including Mil Spec (military grade); ACS, Reagent and Specialized Grade; Food, Horticultural and Drug Grade; Optical Grade, USP, and EP/BP (European Pharmacopeia/English Pharmacopeia), and keeps relevant ASTM testing guidelines. Run-of-the-mill and custom bundling are accessible. Extra specialized, exploration, and security (MSDS) data is accessible similar to a Reference Mini-computer for changing over pertinent units of estimation.
The preparation of Sodium Hydride includes putting the cleaned metallic sodium in steel, porcelain plate, or hard-softened glass tube in fact called a reactor. Preceding the response occurring the air is eliminated from the reactor by passing hydrogen. The metal plate with sodium is warmed to 370o C while the hydrogen utilized is filtered by drying over phosphorus pentoxide and going through the liquefied sodium. The final result of the response which is the sodium hydride can be gathered as it consolidates on the colder pieces of the cylinder as a white plaque.
At the point when Sodium hydride is ready by such a strategy, it contains a limited quantity of metallic sodium as a debasement. One more significant reality about this substance compound is that its unadulterated glasslike white type of it lights at 230o C in an environment of oxygen.
Utilizations of Sodium Hydride
Attributable to its solid base qualities, it tracks down the wide applications in natural and inorganic fine substance arrangements.
It goes about as a strong decreasing and deprotonating specialist for the vast majority of natural responses.
It tracks down a wide application as a desiccant or drying specialist for the majority of natural responses.
Because of its capacity to deliver hydrogen, its road as a hydrogen stockpiling specialist in power device vehicles is being investigated.
Na+H-
A crystalline salt has been synthesized that contains H+ and Na- rather than the usual hydride oxidation states of H- and Na+. The key is irreversible encapsulation of H+ within the cage of 36adamanzane (Adz). The internal proton is kinetically inert to reduction by Na- in solution in NH3−MeNH2 mixtures. Synthesis of the sodide is accomplished by a metathesis reaction between Na and AdzH+X- in which X- is a sacrificial anion such as glycolate, isethionate, or nitrate. Reduction or deprotonation of the sacrificial anion forms insoluble byproducts and AdzH+Na- in solution. After solvent removal, the sodide is dissolved in dimethyl ether and transferred through a frit into a separate chamber for crystallization. The compound was characterized as the sodide by analysis, NMR spectra, and optical absorption spectroscopy.