Sodium amide or sodamide is an inorganic compound with the equation NaNH2. This strong, which is hazardously receptive toward the water, is white when unadulterated, however business tests are normally dim because of the presence of little amounts of metallic iron from the assembling system. Such debasements don't typically influence the utility of the reagent. NaNH2 has been broadly utilized as a serious area of strength for a natural blend.
Sodium amide is ordinarily ready by responding sodium metal with smelling salts, within the sight of an impetus, like iron(III) nitrate. The response is quickest at the edge of boiling over smelling salts, −33 °C.
Na + NH3 → NaNH2 + ½ H2
A decent combination system can be viewed here.
Arrangement and Construction
Sodium amide can be ready by the response of sodium with smelling salts gas,[3] yet it is generally ready by the response in fluid alkali utilizing iron(III) nitrate as an impetus. The response is quickest at the edge of boiling over the smelling salts, c. −33 °C. A terminal, [Na(NH3)6]+e−, is shaped as a response intermediate.[4]
2 Na + 2 NH3 → 2 NaNH2 + H2
NaNH2 is a salt-like material and in that capacity, solidifies as a boundless polymer.[5] The calculation of sodium is tetrahedral.[6] In alkali, NaNH2 structures conductive arrangements, steady with the presence of [Na(NH3)6]+ and NH −2ions.
Utilizes
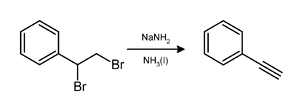
Sodium amide is primarily utilized as an area of strength in natural science, frequently in fluid-smelling salts arrangement. It is the reagent of decision for the drying of smelling salts (fluid or gaseous)[citation needed]. One of the fundamental benefits of the utilization of sodium amide is that it basically works as a nucleophile. In the modern creation of indigo, sodium amide is a part of the profoundly essential blend that prompts the cyclization of N-phenylglycine. The response produces alkali, which is reused ordinarily.
Planning and design
Sodium amide can be ready by the response of sodium with smelling salts gas,[1] yet it is normally ready by the response in fluid smelling salts utilizing iron(III) nitrate as an impetus. The response is quickest at the edge of boiling over of the alkali, ca. - 33 °C.[2]
2 Na + 2 NH3 → 2 NaNH2 + H2
NaNH2 is a salt-like material and thusly, solidifies as a limitless polymer.[3] The calculation of sodium is tetrahedral.[4] In alkali, NaNH2 structures conductive arrangements, steady with the presence of Na(NH3)6+ and NH2-anions.
Security
Sodium amide is destructive and responds savagely with water, delivering smelling salts gas, which is aggravation and poisonous.
Capacity
Sodium amide ought to be put away in impermeable compartments, as Schlenk jars, under dormant gas, similar to argon, and totally away from dampness. Ampouling is an awesome and most secure approach to putting away this compound, particularly for extensive stretches of time.
Delayed contact with air will shape a yellow peroxide, which is shock-delicate and can precipitously explode. Sodium amide tests that are yellow or brown in variety address a serious blast risk.
Removal
Sodium amide can be securely killed by adding it to a huge volume of a drunkard arrangement, with limited quantities of a frail corrosive, such as acidic corrosive, citrus extract, and tartaric corrosive. The corrosive is expected to kill the smelling salt exhaust, yet killing this compound will deliver loads of intensity. Accordingly, the balance arrangement ought to be cooled before use and kept cool during the said cycle. Do this outside.
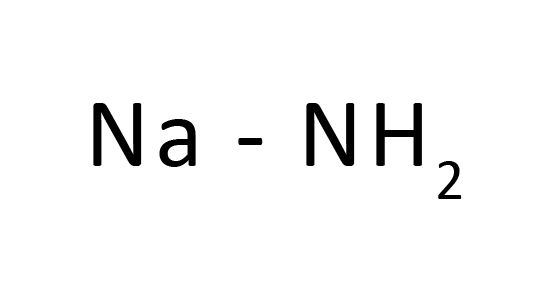
No comments:
Post a Comment