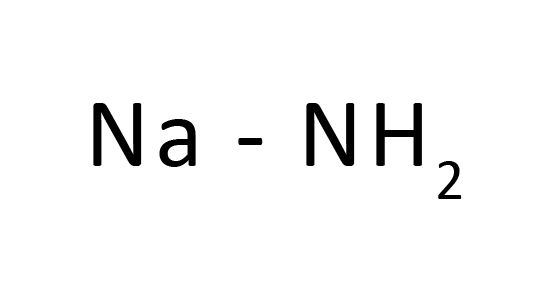Sodium amide, normally known as sodamide, is the chemical compound with the formulation NaNH2. This solid, that's dangerously reactive towards the water, is white while pure, however, industrial samples are generally grey because of the presence of small portions of metal iron from the production process. Such impurities do now no longer commonly have an effect on the software of the reagent. NaNH2 has been broadly hired as a robust base in natural synthesis.
Sodium Amide is mainly used as a strong base in organic chemistry, often in liquid ammonia solution. Sodium amide decomposes explosively in contact with water.
Preparation and structure
Sodium amide may be organized through the response of sodium with ammonia gas, however, additionally, it is organized through the response in liquid ammonia with the usage of iron(III) nitrate as a catalyst. The response is quickest on the boiling factor of the ammonia, ca. -33 °C.
2 Na + 2 NH3 → 2 NaNH2 + H2
NaNH2 is a salt-like cloth and as such, crystallizes as a limitless polymer. The geometry of sodium is tetrahedral.[4] In ammonia, NaNH2 bureaucracy conductive solutions, regular with the presence of Na(NH3)6+ and NH2- anions.
Uses
Sodium amide is used withinside the business manufacturing of indigo, hydrazine, and sodium cyanide. It is the reagent of desire for the drying of ammonia (liquid or gaseous) and is likewise broadly used as a robust base in natural chemistry, regularly in liquid ammonia solution. One of the primary benefits of the usage of sodamide is that it's far an extremely good base and infrequently serves as a nucleophile. It is but poorly soluble and its use has been outmoded through associated reagents together with sodium hydride, sodium bis(trimethylsilyl)amide (NaHMDS), and lithium diisopropylamide (LDA).
Preparation of alkynes
Sodium amide induces the lack of molecules of hydrogen bromide from a vicinal dibromoalkane to provide a carbon-carbon triple bond, as withinside the guidance of phenylacetylene below.
Hydrogen chloride and/or ethanol also can be removed in this way, as withinside the guidance of 1-ethoxy-1-butyne.
Cyclization reactions
Where there's no β-hydrogen to be removed, cyclic compounds can be formed, as withinside the guidance of methylenecyclopropane below.