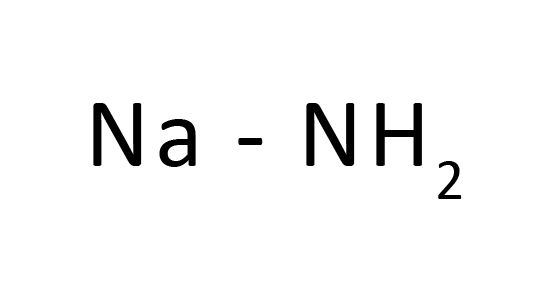NaNH2 (Sodium amide)
What it’s used for: NaNH2 is a strong base. In the rare cases when its strong basicity doesn’t cause side reactions, it can be an excellent nucleophile It’s used for deprotonation of weak acids and also for elimination reactions.
The NH2- anion is the conjugate base of ammonia (NH3). If you’ll recall, the weaker the acid, the stronger the conjugate base – and since NH3 has a pKa of 38, NH2 is a strong base indeed. (Note that although I’m talking about NaNH2 here, the bases LiNH2 and KNH2 essentially behave the same way.)
As a strong base, NaNH2 will deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones.
As a base, it’s often used in situations where a strong, small base is required. Like a piranha, NaNH2 is small, fast, has razor-sharp teeth, and can find its way into tight, enclosed spaces.
Mechanism Of NaNH2: Double Elimination To Give Alkynes
How it works.
Deprotonation of functional groups such as OH and even alkyne C-H should hopefully be straightforward, but the use of bases to make alkenes may require some explanation. This is what is known as an elimination reaction, in that the elements H and Br (in this example) are removed in order to form the alkene. Specifically, this is an example of an E2 reaction.
Since the alkene still has a halide attached, this too can be removed to generate a second double bond (π bond). This is another example of the E2 in that the hydrogen has to be anti to the bromine that is eliminated, but is unusual in that it is sp2 hydrogen that is affected here: