Sodium hydride is a not unusualplace reagent for substrate activation in nucleophilic substitution reactions. Sodium hydride can behave each as a base and as a supply of hydride. This twin capacity withinside the presence of an electrophile which includes benzyl bromide affects withinside the formation of byproducts while dimethylformamide or acetonitrile are used as solvents for those reactions. The structural nature of those byproducts is found in this record.
Sodium hydride is a generally used base for deprotonation of alcohols, phenols, amides, ketones, esters, and different useful corporations for advertising in their nucleophilic substitution.1 Typically, sodium hydride and the reagents are jumbled in polar aprotic solvents which include DMSO, DMF, or acetonitrile for those SN2-kind reactions. Whereas the literature is replete with examples of using those solvents in such reactions, our hazard discovery of reactivity of sodium hydride with those generally used solvents, DMF and acetonitrile, suggests that sure undesired aspect reactions concerning those solvents are probably not unusualplace, however unrecognized. As is disclosed on this record, the foundation reason for those complicating aspect-reactions is the twin position that sodium hydride is famous as a base and as a supply of hydride.
The form of byproducts on the way to be disclosed on this record are usually hard to stumble on with not unusualplace visualization techniques and are smooth to overlook. Using benzyl bromide because the electrophile helped us to isolate the byproducts through UV detection and the systems had been at the end assigned through X-ray evaluation of unmarried crystals.
During benzylation of compound 1 withinside the presence of NaH and benzyl bromide in DMF, we diagnosed an uncommon stable byproduct. The 1H NMR spectrum of this byproduct becomes simple and becomes without resonances originating from the glucal substrate. Assignment of shape becomes achieved through X-ray evaluation. This compound crystallized withinside the monoclinic area institution P21/c. Once in hand, the X-ray shape amazed us, because it confirmed that the stable, compound 3, become a by-product of dimethylamine. At the beginning of the dimethylamine, moiety becomes absolutely DMF, which is served because of the solvent for the response. We are aware that DMF becomes freshly distilled earlier to apply withinside the response and that there has been no hint of dimethylamine in DMF as discerned through a poor ninhydrin assay.
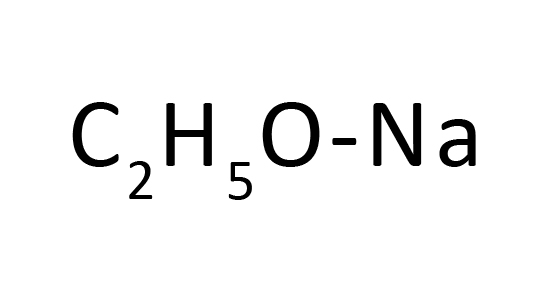
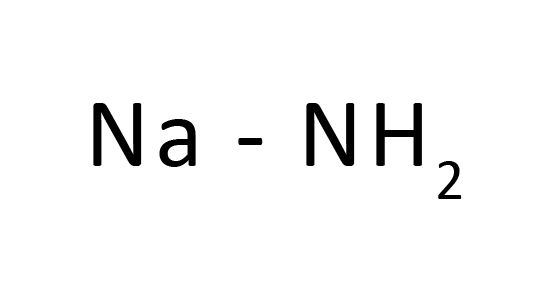
No comments:
Post a Comment