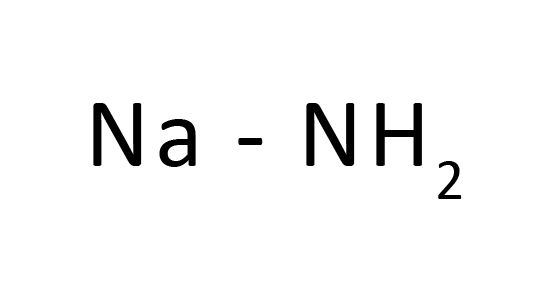Sodium hydride is the chemical compound with the system NaH. It is in general used as a robust base in natural synthesis. NaH is a consultant of the saline hydrides, which means it's miles a salt-like hydride, composed of Na+ and H− ions, in evaluation to the greater molecular hydrides consisting of borane, methane, ammonia, and water. It is an ionic cloth this is insoluble in natural solvents (even though reputedly soluble in molten Na), constant with the reality that H− stays an unknown anion in solution. Because of the insolubility of NaH, all reactions regarding NaH arise on the floor of the strong.
Sodium hydride Formula
Sodium hydride is a pretty reactive inorganic hydride used as a robust base.
Formula and shape: The chemical system of sodium hydride is NaH, and its molar mass is 24. zero g/mol. NaH is an ionic compound as proven below, and is the product of sodium cations (Na+) and hydride anions (H-). It has the equal octahedral crystal shape as NaCl, with every sodium ion surrounded via way of means of six hydride ions. The loose hydride ions deliver this molecule its robust fundamental character.
Preparation: Sodium hydride is ready via way of means of the direct response among sodium metallic and hydrogen gas.
2 Na + H2 → 2 NaH
Physical properties: Pure NaH is drab or white strong with a density of 1. four g/mL, and a melting factor of 800 °C. However, because of its reactivity, its miles normally to be had as a gray strong dispersed in mineral oil (60% weight via way of means of weight) for secure handling.
Chemical properties: Pure NaH can easily ignite in air. When it comes into touch with water gift withinside the air, it releases pretty flammable hydrogen gas. When open to air and moisture, NaH is additionally received without difficulty and hydrolyzed into the robust corrosive base, sodium hydroxide (NaOH). Thus, to save you from fires and explosions, sodium hydride is offered as a dispersion in mineral oil, permitting it to be effectively dealt with in the air.
NaH + H2O → NaOH + H2
Sodium hydride is a robust base usually utilized in natural chemistry, in which its miles are stored in an inert atmosphere (with 'dry' gases consisting of argon, nitrogen, etc.). It is insoluble in natural solvents.
Uses: Despite its risky reactivity, NaH is extensively used as an effective deprotonating and decreasing agent for plenty of natural reactions. It also can be used as a desiccant or drying agent for laboratory chemicals. Due to its cap potential to launch hydrogen gas, its miles are being explored as a hydrogen garage agent in gas molecular vehicles.