Sodium hydride is the chemical compound with the empirical system NaH. This alkali metallic hydride is by and large used as a robust, but flammable base in natural synthesis. NaH is a base of extensive scope and application in natural chemistry. As a superbase, it's miles able to deprotonate various even susceptible Bronsted acids to provide the corresponding sodium derivatives. Typical "easy" substrates incorporate O-H, N-H, and S-H bonds, inclusive of alcohols, phenols, pyrazoles, and thiols.
About:
Sodium Hydride is largely an alkali metal hydride with the empirical system of NaH. This chemical compound is taken into consideration as a robust base that is broadly utilized in natural chemistry. Sodium hydride is likewise taken into consideration as saline hydrides or salt-like hydrides due to the fact it's miles composed of Na+ and H- ions in contrast to the opposite molecular hydrides which include methane, ammonia, etc. It is rather insoluble in natural solvents and thanks to these belongings all reactions regarding NaH arise on the floor of the solid.
Preparation of Sodium Hydride
The education of Sodium Hydride includes the setting of the wiped clean metal sodium in steel, porcelain tray, or a hard-melted glass tube technically referred to as a reactor. Prior to the response-taking region, the air is eliminated from the reactor through passing hydrogen. The metallic tray with sodium is heated to 370o C whilst the hydrogen used is purified by drying over phosphorus pentoxide and passing it via the melted sodium. The quit made from the response this is the sodium hydride may be gathered because it condenses at the chillier elements of the tube withinside the shape of a white plaque.
When Sodium hydride is ready through such an approach it incorporates a small quantity of metal sodium as an impurity. Another crucial reality approximately this chemical compound is that the natural crystalline white shape ignites at 230o C in an ecosystem of oxygen.
Uses of Sodium Hydride
Owing to its robust base characteristics, it reveals extensive utility in natural and inorganic great chemical education.
It acts as an effective lowering and deprotonating agent for lots of natural reactions.
It reveals an extensive utility as a desiccant or drying agent for lots of natural reactions.
On account of its cap potential to launch hydrogen, it is the road as a hydrogen garage agent in gasoline molecular cars is being explored.
Na+H-
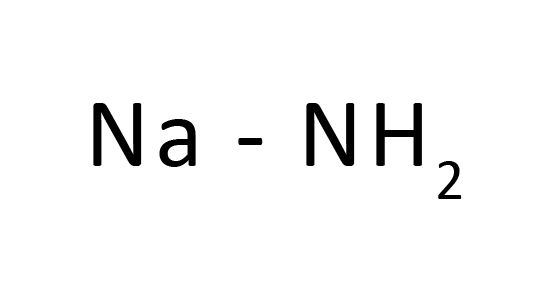
No comments:
Post a Comment