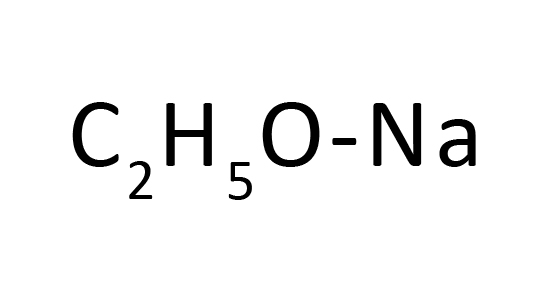4,4'-Bipyridine is used in transition-metal complex catalyst chemistry for uniform polymerization, luminescence chemistry, and in spectrophotometric analysis. It plays an important role as a photosensitizer and luminescent material. It is also used as a precursor to paraquat viz. N,N'-dimethyl-4,4'-bipyridinium.
This Thermo Scientific brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product/item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific.
Applications
4,4′-Bipyridine is used in transition-metal complex catalyst chemistry for uniform polymerization, luminescence chemistry, and in spectrophotometric analysis. It plays an important role as a photosensitizer and luminescent material. It is also used as a precursor to paraquat viz. N, N′-dimethyl-4,4′-bipyridinium.
Chemical Structure Description
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The 4,4'-Bipyridine molecule contains a total of 21 bond(s) There are 13 non-H bond(s), 12 multiple bond(s), 1 rotatable bond(s), 12 aromatic bond(s), 2 six-membered ring(s) and 2 Pyridine(s).
Images of the chemical structure of 4,4'-Bipyridine are given below:


The 2D chemical structure image of 4,4'-Bipyridine is also called the skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of 4,4'-Bipyridine are implied to be located at the corner(s) and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
The 3D chemical structure image of 4,4'-Bipyridine is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between them. The radius of the spheres is, therefore, smaller than the rod lengths in order to provide a clearer view of the atoms and bonds throughout the chemical structure model of 4,4'-Bipyridine.
The 4,4'-Bipyridine molecule shown in the visualization screen can be rotated interactively by keep clicking and moving the mouse button. Mouse wheel zoom is available as well – the size of the 4,4'-Bipyridine molecule can be increased or decreased by scrolling the mouse wheel.
The information of the atoms, bonds, connectivity, and coordinates included in the chemical structure of 4,4'-Bipyridine can easily be identified by this visualization. By right-clicking the visualization screen, various other options are available including the visualization of van der Waals surface and exporting to an image file.